When you absolutely have to recruit patients on time
Home Success Stories Top Recruiters in Ulcerative Colitis Phase III Study
Top Recruiters in Ulcerative Colitis Phase III Study
Investigational Product: Amino Salicylate Anti-inflammatory
Therapeutic Area: Gastroenterology
Indication: Ulcerative Colitis
Study Phase: III
Sponsor: Pharmaceutical Company
Study design: Double-blind, double-dummy, randomized, multi-center, comparative Phase III clinical study
Total number of patients: 306
Sponsor’s goals: To expedite patient enrollment into the clinical trial to substantially reduce the time for the drug to enter the market.
Our solution: Eastern Europe and Russia have been proven to act as some of the top recruiting geographies in the past. We leveraged the centralized healthcare system and dense populations to tap into the patient pool and drive recruitment forward. We selected Russia, Ukraine, Latvia, and Lithuania.

Case Study Metrics
Patient Recruitment by Accell
| Russia | Ukraine | Latvia | Lithuania | Accell Total | Overall Study Total* | |
|---|---|---|---|---|---|---|
| Enrollment Period (months) | 10 | 5 | 14 | 14 | 14 | 14 |
| Active Sites | 13 | 6 | 4 | 4 | 27 | 41 |
| Patients Enrolled | 122 | 73 | 34 | 29 | 258 | 306 |
| Enrollment Rate (patients/site/month) | 0.94 | 2.43 | 0.61 | 0.52 | Accell's Average: 1.13 | Study Average: 0.74 |
* Investigational sites in Germany, Hungary, and Poland also participated in this clinical study.
Ukrainian investigational sites achieved notable patient recruitment rates due to a large number of patients meeting the protocol-specific criteria, highly organized and well-planned work of the investigational teams at sites, and close support and management by Accell’s clinical staff.
Average Number of Patients per Site per Month
- Accell’s Countries – 1.13
- Rest of the World – 0.23
% Patients Randomized per Country
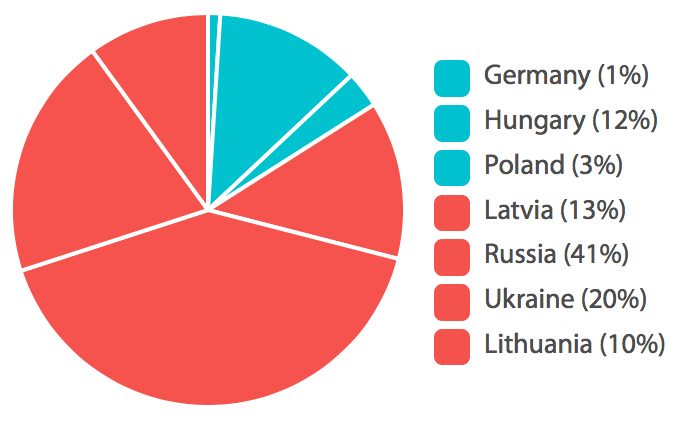
Accell recruited 84% of total patients for this clinical trial, with only 65% of a total number of study sites. Two Russian sites were sponsor-audited, and one Lithuanian site was subject to a regulatory inspection, all without critical findings. The Sponsor was able to end recruitment earlier than planned, as the interim analysis goals were met. Fast recruitment and the reduction of the number of active sites can ultimately lead to a reduction of direct and indirect costs of clinical research, making Eastern Europe and Russia attractive regions for clinical study conduct.
Success Stories
our SERVICES MENU
From clinical research associates to hands-on founders, we fiercely pursue our strategic goal: deliver on schedule. We think of ourselves as your development partner, not a vendor.
